Local Innovation, Global Impact: Clarity Medical's EtCO2 module, Since 2007
Since time immemorial, inventions and cutting-edge research by Indian scientists have helped transform the way we lead our lives. Indian innovations in metallurgy, mathematics, sciences, astronomy, and medicine helped India solidify its position as an innovator globally. From the invention of buttons in the Indus Valley civilization to Rhinoplasty by Surushta in 600 BC, India has been at the forefront of scientific research and innovation globally.
Today, India's home-grown medical device manufacturers are developing cutting-edge products that cater to the unique needs of the Indian market. Additionally, international medical device companies are partnering with Indian firms to develop products that are affordable and accessible to patients worldwide. With its focus on innovation, India is poised to play a major role in the global medical product development industry.
India, EtCO2, and respiratory care
A major component of India’s disease burden is its reliance on imported diagnostic tools to provide quality care. India imports most of its respiratory care and diagnostic devices as few homegrown manufacturers exist. Although common devices such as SpO2 monitors and other diagnostic devices exist in India, hospitals and medical product companies still look to the West for specialized devices such as EtCO2 modules. This costs the the Indian healthcare industry millions of dollars that could easily be saved if this equipment is manufactured locally.
What is EtCO2?
EtCO2—or end-tidal carbon dioxide is the partial pressure of carbon dioxide (CO2) in exhaled breaths. ETCO2 measurement is used in medical settings to assess a patient's ventilation and respiratory status. ETCO2 monitoring (capnography) is an invaluable tool for assessing respiratory function and identifying potential respiratory complications in non-intubated and intubated patients.
In non-intubated patients, ETCO2 monitoring can help to identify respiratory distress, such as in cases of asthma, chronic obstructive pulmonary disease (COPD), or COVID-19. EtCO2 is also used to monitor patients during sedation, anaesthesia, or cardiopulmonary resuscitation (CPR).
Why is EtCO2 better for monitoring respiratory distress?
Respiratory distress causes an increase in carbon dioxide in the body. However, if clinics or hospitals only focus on SpO2 levels, unexpected downturns can complicate things quickly—and often without warning. EtCO2 values measure the patient's carbon dioxide (CO2) values. These values are stable and a highly accurate reflection of the patient's ventilatory and perfusion/metabolic status—leading to better disease management.
Addressing the need for local R&D in EtCO2 technology manufacturing.
As a developing, cost-conscious country, India is gradually increasing its healthcare expenditure in public hospitals. However, there are many reasons for the low repair or even replacement rates of specific technologies and diagnostic or monitoring aids such as EtCO2. Some of the reasons for this include:
>High costs and import duties
Taxes and import duties can drive up the costs of imported equipment. This is also true for EtCO2 monitors, as most are imported from other countries.
>Dependency on imports
Very few companies put in the time and resources required for innovation and R&D, leading to an over-reliance on imports—and higher costs.
>Water trap
The air we expire also contains water vapour. Water trapping causes moisture to accumulate on or around the EtCO2 IR sensor (IR Cell), leading to false highs and reducing its efficiency and capability to monitor the patient's ventilation and overall respiratory status.
>Sensor damage
Water vapour can also damage the sensor permanently, causing equipment failure and lowering the hospital's patient care standards. Additionally, the lack of funds can further impact getting replacements for damaged machines or sensors.
>High respiration rates for accurate analysis
Some EtCO2 machines needed higher respiration rates for accurate analysis. This impacted the accuracy of EtCO2 readings in patients with compromised respiratory systems or lower respiratory rates.
Clarity Medical—driving India towards self-reliance in medical diagnostics
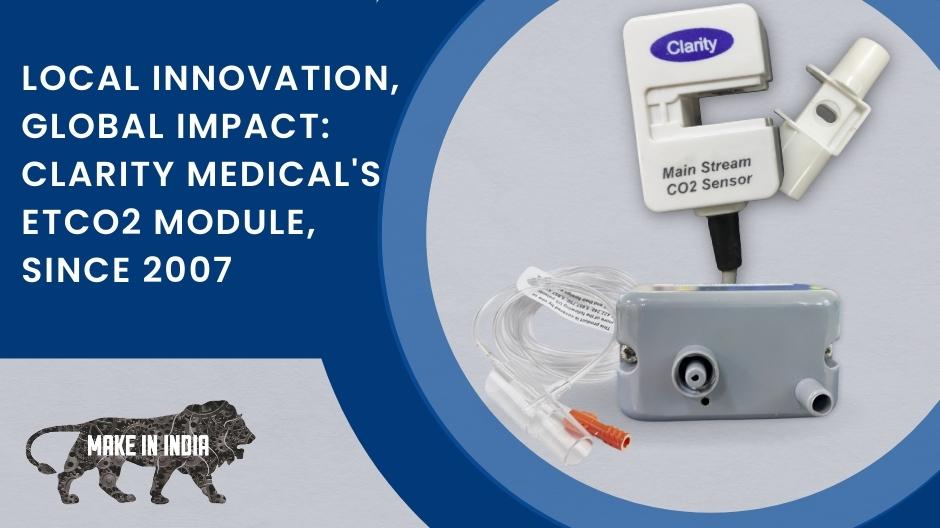
Clarity Medical understood the market need for a scientific Make in India enterprise and focused its R&D on building a homegrown EtCO2 module. Clarity launched India’s first indigenous EtCO2 module in 2006 that works seamlessly with its patient monitors and those of other brands.
While most other vendors integrate EtCO2 modules from companies such as CPT, Respironics, and PhaseIN, Clarity Medical is leading the way in the total indigenization of their products with homegrown products and services.
Clarity Medical’s EtCO2 modules - Made in India - for the world
Clarity benchmarks its indigenously developed EtCO2 module against top EtCO2 modules to showcase its reliability. This benchmarking showcased the following benefits:
>No water trapping
A unique airway adapter reduces water trapping to the minimum. This reduces the impact of vapour on the detector and sensor, helping to get accurate EtCO2 values every time.
>Reduced false readings
There is virtually no vapour accumulation on the sensor, so the chances of reading errors are minimized.
>Highly accurate low-flow calibration
A high-accuracy low-flow calibration allows medical professionals to get accurate results even if the patients have a low respiration rate, leading to world-class levels of patient care.
>Compatibility
Clarity Medical designed India’s first EtCO2 module to be highly adaptive and compatible with various patient monitors. This allows hospitals to reduce expenses and provide high- quality patient care.
Conclusion
The first step towards empowering healthcare facilities in any country is to ramp up localized production of quality diagnostic equipment. Although India is a hub for producing world-class, high-quality medical devices, some specialized modules are still imported from other countries, increasing their acquisition cost. Clarity Medical works upon India's need to manufacture international-grade diagnostic equipment and is a leading name in indigenous EtCO2 modules, EMG devices, MG/NCV/EP devices, pulse oximeters, remote patient monitors, and other ground-breaking telemedicine solutions. Clarity Medical's trusted devices are used in public and private hospitals in India and worldwide.